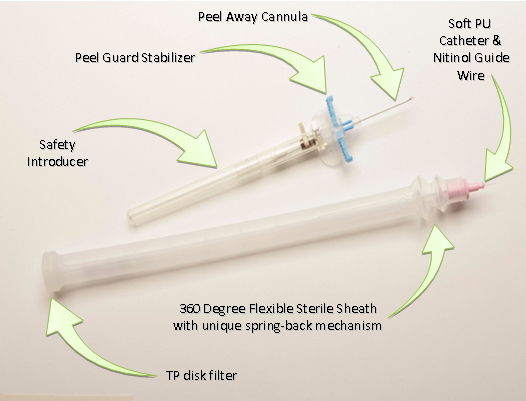
Flexicath M/29® Midterm® catheter incorporates 4 different technologies that allow a smooth insertion with complete sterility throughout the process.
It starts with the Peelguard® introducer that enables the splitting of the OTN split able cannula without the need of extracting it first. It prevents the need to handle the catheter that is going through the cannula, add safety and ease of use to the process. The Peelguard® also enable the attachment of the catheter unit tip to the introducer, keeping the process completely closed and prevent any exposure of the catheter while performing the procedure.
The next two features that make the whole process sterile are the Silicon sterile barrier and the special developed TP filter.
The Silicon sterile barrier prevents from any contact between catheter and the patient skin, the user hands and other contaminates. Its geometry prevents it from collapsing onto the catheter and blocking the movement but still remain soft enough to keep the users hand in control without losing the “feel” of the insertion.
The TP filter, is especially design filter that allows the transfer of pressure that is created while changing the volume of the sleeve to the environment while still keep the system close to prevent any contact with the environment. The filter radical design allows the smooth operation even if there is accidental release of fluids into the sleeve.
To allow the smooth advancement of the catheter through the sleeve without complications and without losing the feel, the M/29® incorporates the bellow bounce back mechanism.
The Bounce back mechanism is a spring like bellow that allows the Silicone sterile barrier to move back after a portion of the catheter is inserted without the need of any user involvement. It creates a smooth insertion procedure will keeping the whole process in the sleeve. This feature is essential for the mobility of the catheter and prevents the need of the operator to pull the sleeve back to the opposite direction of the insertion.
In a Category All By Itself
The M/29® offers a new, cost-effective standard in comfort, safety and productivity specifically for the midterm® category. Designed with both patients and caregivers in mind, it dramatically reduces insertion preparation and complexity.
The M/29® is an ideal solution for those patients receiving peripheral IV therapy from one to four weeks and can be inserted safely and effectively in 10 minutes, or less by trained clinical staff.
The M/29® is the first and only catheter system specifically designed to eliminate both airborne and touch contamination during placement and reduce the associated risks of catheter related blood stream infections.
The safety introducer and soft polyurethane catheter, with its flexible sterile silicone sleeve, combine to minimize the potential for both airborne and touch contamination during placement.
The peel away cannula and Peelguard® stabilizer prevent vessel trauma and catheter migration during the final steps of placement.
Technology Highlights
- Revolutionary advancement of traditional midline catheter
- Pressure rated to 300 PSI and 5ml/Sec
- The first product in a platform of innovative vascular access devices
- Only FDA cleared midline designed to control infection from start
- Designed with both patients and caregivers in mind
- Simple to place, intermediate dwell alternative to both traditional midline catheters and short peripheral catheters
- Contamination blocking – built in sterile field
- Reduces insertion preparation, complexity, and cost
- Improves patient care
- Existing reimbursement and catheter insertion protocols
