The M/29® Midterm® Catheter System. An innovative platform for care
The M/29® was designed to answer an obvious need for a safe, easy-to-insert catheter for intermediate dwell applications. The M/29® is the only touch-free midline that can be placed in under 10 minutes following minimal training. We set out to revolutionize intermediate dwell IV therapy with the world’s first easy-to-place, touch-free midline catheter. Midlines no longer need to be tedious, costly and complicated procedures – the simplicity of short peripheral placement with true midline performance.
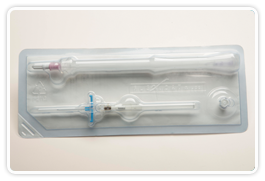

- Simple to place like a short peripheral catheter
- Over the needle insertion
- Minimal training required for competency
- Build-in 360 Maximum Barrier®/3D Maximum Barrier®
- Reduces multiple needle sticks
- Requires no X-ray after placement
- Made of biocompatible radiopaque polyurethane
- Premium Nitinol Guidewire
- Latex-free components
- Needle-stick safety components
- Eliminates risk of airborne or touch contamination
- Indwelling time > 29 days
- Applicable for most common infusates
- Peelguard® eliminates catheter migration
- Pressure rated to 300 PSI and 5ml/Sec
- Cost effective for therapies in excess of 4 days
Features
The M/29® Midterm® — perfect for intermediate-term I.V. therapies
- Color-coded components – identify the type of catheter and size, which advance patient safety and mean less confusion
- Simple to place like a short peripheral catheter - does not require modified Seldinger technique like conventional midlines and PICC’s – reducing training time
- 360 degree Flexible Sterile Sheath with integral spring back mechanism eliminates risk of airborne or touch contamination
- Minimal draping – does not require maximal barrier
- Requires no X-ray after placement
- Made of biocompatible flexible polyurethane and premium Nitinol Guide wire
- Latex-free components – provide options for clinicians/patients with latex allergies
- Needle-stick safety components – enhance clinician/patient safety and ensure hospital compliance with the federal safety regulations
- Peelguard® eliminates catheter migration, prevent trauma and simplify procedure
- Pressure rated to 300 PSI and 5ml/Sec
- Cost effective for therapies in excess of 4 days
Specifications:
- Catheters: Polyurethane, pressure rated to 300 PSI, 5ml/Sec
- Introducer: PTFE 3F
- 360 Maximum Barrier®: Silicone
- Peelguard®: Silicone
- Guidwire: Nitinol
M/29® 3F10 Specifications:

- Catheter material: Polyurethane
- Guide wire: Nitinol
- Catheter Length: 10cm (4 inches)
- Radio opaque: yes
- Flow: 12ml/min
- Color coded hub: Pink
- Sleeve material: Medical grade Silicone
- Introducer needle: 19G
- Pressure: Up to 300psi (or 5 ml/sec max viscosity 12 cP)
M/29® 3F20 Specifications:
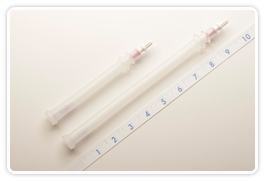
- Catheter material : Polyurethane
- Guide wire: Nitinol
- Catheter Length: 20cm (8 inches)
- Radio opaque: yes
- Flow: 12ml/min
- Color coded hub: Pink
- Sleeve material: Medical grade Silicone
- Introducer needle: 19G
- Pressure: Up to 300psi (or 5 ml/sec max viscosity 12 cP)
M/29® Peelguard® Introducer Specifications:
- Introducer sheath: PTFE
- Needle size: 19G
- Needle effective length: 32mm
- PeelGuard® material: Medical grade Silicone
- Safety mechanism type: active
